In This Issue...
Have a story you want to share or a topic you would like us to cover?
Jeff Weighs In
Advocacy and Legal Team – Legislative and Regulatory Updates
by Artemis Policy Group
2025 – An Eventful Year in Health Policy and Advocacy
2025 brought sweeping changes to the political environment at an intensely quick pace, including many which impact hemophilia treatment centers (HTCs) and people with bleeding disorders. With these new challenges also came opportunities for the bleeding disorders community to stand together.
The year began with President Trump issuing a record number of 142 executive orders in just 100 days and articulating a new approach to federal funding for health care. Initially, we prepared for extensive cuts and a reorganization of the Department of Health and Human Services, including bleeding disorders activities, into the Administration for a Healthy America (AHA). Instead, we survived the longest government shutdown in U.S. history, lasting 43 days. Ultimately, we did not see the creation of AHA and Congress passed a continuing resolution to fund the federal government at existing funding levels until the end of January 2026, but this is only a temporary solution. As such, we anticipate advocacy for federal funding to continue.
Despite happening during the government shutdown, the Alliance was able to hold its biggest ever Hill Day on October 7th with over 130 participants attending 10 member-level meetings with Senators or Representatives, and 111 meetings with Hill staff. We focused not just on the importance of the 340B Program to HTCs and our patients but also advocated for the federal programs and for Congress to address barriers to insurance and care that our patients are facing.
The summer was dominated by passage of H.R. 1, the “One, Big Beautiful Bill Act,” using a congressional tool known as budget reconciliation. H.R. 1 made the largest cuts to Medicaid in the program’s history, and will over time reduce Medicaid eligibility and enrollment, as well as limit state financing mechanisms. With implementation of H.R. 1 underway and the anticipated expiration of tax credits for Marketplace plans at the end of 2025, we expect access to insurance will remain an important focal point of advocacy and compliance work as HTCs grapple with reimbursement changes and the entire community works to ensure people with bleeding disorders have access to high-quality insurance coverage.
The Alliance team also continued work to address other insurance barriers, particularly around affordability, including leadership in the All Copays Count Coalition and Alternative Funding Taskforce, and providing comments on HHS-Labor-Treasury RFI on drug pricing.
2025 brought significant activity on the 340B Discount Drug Pricing program in terms of new legislative proposals, congressional hearings, and a Congressional Budget Office report on growth trends in the program. We also saw the Trump Administration approve a pilot program, testing a rebate model for the 340B program.
Many of these issues will persist into 2026. The good news is that the bleeding disorders community has strong friends on both sides of the aisle in Washington, and a compelling story to tell. The national bleeding disorders organizations, with substantial leadership from the Alliance, will continue to work together on strategies to protect people with bleeding disorders and the HTCs that serve them.
We end the year (and this article!) with huge thanks to all Alliance members and partners who educated and advocated this year on behalf of the community. Thank you for reading our many eblasts, emailing and calling your Members of Congress and state legislators, spreading the word to your colleagues and friends, and always showing up for this community. Wishing everyone a very happy and restful holiday season – and we’ll see you back in the fight in January!
Ambetter Insurance Plans – Prepare Your HTC and Patients for 2026
Hemophilia Alliance staff recently learned that Ambetter, a Centene-owned health insurer that offers Marketplace plans in 29 states, has published a very pared-down formulary for its 2026 health plans (applicable in almost all of the states where it does business).
- The published Ambetter formulary lists only Afstyla, tranexamic acid, and aminocaproic acid. It includes no other bleeding disorders medications: nothing for the treatment of entire disease states (e.g., hemophilia B, hemophilia A with inhibitors, von Willebrand disease, etc.) and no non-factor (e.g. injection) products.
- A separate Ambetter document details changes from the 2025 formulary, noting the removal of 11 hemophilia agents (click here to download pdf).
- Ambetter sent those documents to patients who are currently enrolled in Ambetter coverage, along with letters informing the patients that their current medication “will no longer be covered.” The letter encouraged patients to talk with their doctor about other options OR submit a request for an exception AFTER December 31, 2025.
The Hemophilia Alliance and other community members have engaged with Ambetter to voice our concerns over the published formulary and Ambetter’s communications with currently-enrolled patients. We have learned:
- Ambetter will provide a pathway for patients and providers to request prior authorization for medically necessary non-formulary medications.
- Prior authorization processes and forms remain the same, but submissions will not be accepted before January 1, 2026.
- Health care providers can access Ambetter’s clinical policies for affording access to medically-necessary bleeding disorders products at this hub: Clinical & Payment Policies. Click on the drop-down menu for “Pharmacy and Biopharmacy Policies” for links to pdf downloads for
- Emicizumab-kxwh (Hemlibra)
- Factor IX and Factor IX Complex
- Factor VIIa, Recombinant
- Factor VIII
- Factor VIII/von Willebrand Factor Complex
- Factor XIII (Recombinant and Human)
What HTCs can do now. Please be aware of this issue. Your patients with 2025 Ambetter coverage may wish to explore additional/other coverage options for 2026. Some patients who seek to continue with Ambetter coverage in 2026 may have received 12-month prior authorization (PA) approvals that extend beyond January 1, 2026; those patients or their care teams should check whether Ambetter will continue to honor the PAs for the full 12-month terms. Care teams could consider preparing now so they can submit PA requests as early as possible in the new year.
Update on Medicare Reimbursement Issues for Some Bleeding Disorders Treatments
The Hemophilia Alliance has heard from a couple of member HTCs that have received denials from Medicare on claims for at least one of the newer non-factor hemophilia treatments that are administered by injection. While Hympavzi, Qfitlia, and Alhemo have all received J-codes and have been designated by Medicare as “clotting factors,” most of the local Medicare Administrative Contractors (MACs), the entities that pay Part A and B claims, have not updated their Part B clotting factor billing and coding articles to include the newer treatments.
Further research (see here) of Medicare MAC policies is showing that only Hemlibra is consistently listed as a Part B drug, and claims are paid by the Medicare Part A/B MACs. Some of the MACs are mis-categorizing these new products as Durable Medical Equipment (DME) and as such, are requiring that the drugs be paid by the DME MAC contractors and will only pay for treatments that are administered “incident to a physician service” and are denying claims for treatments that are self-administered. Other MACs are silent on these new products.
The Hemophilia Alliance plans to educate all MACs about these new drugs, explaining that CMS is treating the newer injections as if they are “clotting factors,” and as such should be considered Part B drugs and included in their Part B policies for clotting factors, which allow for self-administration.
In the meantime, if you are currently dispensing Hympavzi, Qfitlia or Alhemo to any Medicare beneficiaries, the Hemophilia Alliance encourages you to reach out to your Primary Member and Community Relations (MCR) contact. We will work with you to address any billing issues as we work to get the policy fixed nationwide.
Please contact your Primary MCR contact with any questions.
Member and Community Relations Update
The Member & Community Relations Team in 2025
by Jennifer Borrillo, Senior Vice President Member & Community Relations
The Hemophilia Alliance Member and Community Relations (MCR) team is comprised of Directors Zack Duffy, Angie Blue, Karen Bowe-Hause, Mark Plencner, Jazzmine Brown, Ashley Castello, and Kelly Waters; they report to the Senior Vice President of Member & Community Relations, Jennifer Borrillo. This passionate and dynamic team is pivotal in supporting the mission of the Hemophilia Alliance, ensuring that member Hemophilia Treatment Centers (HTCs) have the expertise, resources, and public support to sustain their integrated clinical and pharmacy services for individuals with bleeding and clotting disorders.
Throughout 2025 some of the MCRs’ key efforts in supporting member HTCs included:
- Daily Operations & Strategic Guidance: Advising and consulting with external partners and member centers, providing guidance on daily operations, and supporting centers in patient access to care.
- Stakeholder Engagement: Meeting regularly with HTCs, chapters, payers, brokers, community partners, and institutional personnel to improve, educate, and support their efforts.
- Program Development: Identifying opportunities for HTCs to dispense medications directly to patients, including through the Hemophilia Alliance Network Services (HANS), single case agreements, and insurance payer and PBM contract negotiations.
- Regulatory Expertise: Maintaining up-to-date knowledge of the 340B Program rules, regulations, and potential changes impacting members.
- Collaboration Initiatives: Developing and implementing programs with national patient organizations to enhance relationships between HTCs, state and local patient organizations, and the broader community.
- HTC Sustainability: Reinforcing HTC sustainability by supporting member HTCs in growing their pharmacy programs, participating in managed care and commercial clinical/pharmacy contracting, and engaging in federal and state public payer initiatives.
- Operational Support: Collaborating with the Hemophilia Alliance GPO, Advocacy, Payer Relations, Membership, and Communication teams to provide operational support and assistance as needed.
Several 2025 MCR initiative highlights include:
Team Expansion and Transition – In 2025, the MCR team found itself in a state of transition: planning to add a new Director position to the team, adjusting to the departure of a team member in July, and proactively preparing for another Director’s pending retirement in January 2026. The team remained focused on maintaining continuity and building strong relationships with their member HTCs and excitedly welcomed three new Directors in September 2025. The team has been thoughtful in the onboarding of the new team members and preparing each HTC for these transitions.
Community Engagement and Event Participation – The MCR team attended numerous national, state, and regional events, and meetings in more than 20 states: conferences, advocacy events, regional meetings, HTC visits, and more. They had a presence at organizational meetings sponsored by partners such as NBDF, HFA, Coalition for Hemophilia B, HTRS, ATHN, VWD Connect, and the Self-Insurance Institute of America (SIIA). The team’s presence at these events was aimed at supporting the bleeding and clotting disorder community, sharing resources, and strengthening partnerships with HTCs and community organizations.
Advocacy and Education – The MCR team played a key role in education and advocacy efforts, including educating payers, brokers, consultants, employers, and government agencies about the value of member HTCs as Centers of Excellence and the critical importance of the bleeding disorders community having access to care at Federally Designated HTCs. The MCRs participated in payer education initiatives to strengthen HTC knowledge and effectiveness with payers and engaged in collaborative initiatives with patient advocacy organizations.
Member Support and Communication – The MCRs served as the primary point of contact for member HTCs, triaging questions, assessing next steps, assisting with strategic discussions, developing resources for member HTCs, and helping with future planning, growth, and sustainability. Proactive communication was emphasized throughout the year, and member HTCs were encouraged to reach out early with questions or issues to enable timely intervention and support.
Monitoring and Assessment – The MCRs consistently engaged with member HTCs on issues, concerns, and questions, monitored threats to patient access to care and proactively worked with HTCs and community partners as needed, assessed potential growth opportunities for member HTC pharmacy programs, and worked diligently with member HTCs to ensure ongoing individualized approaches to sustainability and effectiveness.
Impact and Significance – The MCRs have been instrumental in strengthening the operational capacity and sustainability of member HTCs, expanding the reach and effectiveness of the Hemophilia Alliance through strategic partnerships and advocacy, enhancing communication and support for HTCs and the broader bleeding disorders community, and navigating regulatory changes and payer dynamics to ensure continued access to high-quality care and resources for the bleeding disorders community.
Remember the Member & Community Relations team WORKS FOR YOU and we look forward to engaging with you in 2026. Let us know how we can help you!
Understanding Individual Coverage Health Reimbursement Arrangements (ICHRAs)
by Angie Blue, Director of MCR and Roland Lamy, VP of Payer Relations
An Individual Coverage Health Reimbursement Arrangement (ICHRA) is an innovative model whereby employers discontinue traditional group coverage and replace it with a Health Reimbursement Account (HRA) that provides employees with funds to purchase their own individual coverage. The ICHRA model has gained significant traction in the market in recent years and may be particularly attractive to employers that have employees that are considered “high cost” or “high risk,” like Hemophilia Treatment Center (HTCs) patients with bleeding disorders. Employers argue that ICHRAs allow employees to select plans that best meet their needs, whether through the ACA marketplace, directly from insurers, or via Medicare if they qualify. The Alliance team has heard from a few HTCs with questions about ICHRA plans. While we know the prevalence of this model varies across regions, it does appear that more employers are turning to ICHRA plans for 2026.
For employees with bleeding disorders, it is critical to consider whether the benefits and coverages available to them via ICHRA provide adequate coverage for their condition. We know that individual plans sold directly in certain markets or through marketplace exchanges vary greatly in three important areas. They vary in terms of bleeding disorder product coverages. They vary in terms of cost-sharing, including copayments, deductibles, and out-of-pocket maximums. They vary in terms of network access – critically, whether the HTC is included in-network for both clinical care and clotting factor dispensations.
Individuals who are being shifted from group coverage to ICHRA, in short, will have to do substantial research to ensure that they pick an individual plan that will meet their needs – more than they may have done when they simply received coverage under the group plan. Whatever health plan the individual considers, they should be sure to ask questions about product and benefit coverages and network access to the HTC. Some brokers and employers will “encourage” employees to choose a particular ICHRA sponsored plan without perhaps knowing or understanding the specific health issues facing the employee. It is critical that the employee compare ICHRA Plans across the three key areas – formulary, cost-sharing, and network – to ensure that their chosen plan meets their needs.
With respect to network access, individual plans, whether sold on or off the marketplace exchange, often have more restrictive network access or unique network access rules. For example, a resident of New Hampshire who previously had a group plan that allowed access to Boston providers may find that an ICHRA plan does not offer the same network access. The employee should be sure to inquire about network access for BOTH the clinical services and clotting factor products.
Lastly, it is crucial to inquire about the process of switching between ICHRA Plans offered in the market in the event of an adverse mid-year change in coverage. We know from hard experience that some health plans change their rules mid-year – for example, establishing new rules on product coverages or new rules on network access. Employees should be aware of any policies, rules, or subtle benefit differences that might affect their ability to adjust and change plans mid-year based on a “qualifying event” like network access to their HTC provider. Marketplaces may vary with respect to mid-year plan changes, so it is a good practice for those choosing individual health plans to ask the right questions and understand whether there are options to make a mid-year change or not.
By thoroughly evaluating these aspects, patients can make more informed decisions about their health coverage under an ICHRA and choose a plan that best meets their needs.
Administration and Operations Update
2025 – A Year In Review
by Administration & Operations Team
The Alliance had a busy 2025. We hosted 7 member meetings and 23 webinars, communicated 39 unique eblasts, and distributed 6 newsletters- keeping our community informed and connected. The Alliance is deeply grateful to our industry partners for their support,  and to our planning committees—comprised of dedicated member experts—whose passion and creativity helped make each of these activities exceptional.
and to our planning committees—comprised of dedicated member experts—whose passion and creativity helped make each of these activities exceptional.
- Of the seven member meetings, four offered CEUs to our member Pharmacists, Social Workers, Physical Therapists and Nurses. These gatherings showcase clinical best practices with a welcoming space where knowledge is shared and lasting connections are formed.
- The New HTC Staff Meeting equipped new team members with essential training in operations, payer relations, regulatory updates, and advocacy – all in support of the comprehensive, patient-centered care model.
- Our Spring and Fall Member Meetings continue to bring value to our members through networking, updates, and the opportunity to discuss and brainstorm solutions to common challenges. For the Fall Member Meeting & Hill Day in Washington, DC, we united with patients and families to educate our legislators about bleeding disorders and the value of the HTC model. The event proved impactful despite the government shutdown. We logged many steps and executed the highest number of in-person meetings with our representatives to date.
In 2026, we will be hosting 8 member meetings, beginning with the MPBA Meeting in Newport Beach, CA. With new tools for travel booking and event registration, plus a Member Needs Assessment, we look forward to advancing our mission and strengthening our community—be on the lookout for your chance to share feedback!
Happy Holidays and See You in the New Year!
Notes From The Community
Spring 340B Grantees Conference
by Jeff Blake, Heidi Lane and Jennifer Borrillo
Hemophilia Alliance senior leadership is excited to share that for the first time we will be coordinating efforts with the Ryan White Clinics for 340B Access (RWC-340B) on planning sessions at their Spring 2026 Grantees Conference for the first time.
RWC-340B and their conference partners are inviting all Federal Grantees, including organizations such as Ryan White Clinics, Federally Qualified Health Centers (FQHCs), and specialty clinics including Tribal, Black Lung, and HTCs, to attend the conference. The conference curriculum holds value for every grantee and staff member, regardless of role or background.
The Spring 2026 Grantees Conference will take place in Houston, Texas, from May 28-May 30, 2026, at the Hyatt Regency Houston Downtown. Preconference education will begin on May 28th, with the nationally acclaimed 340B University, sponsored by Apexus, the Prime 340B Vendor. Two full days of plenary and breakout sessions will follow, covering issues of concern to grantees, such as fiscal management, audit and compliance, advocacy, pharmacy management, use of TPAs, and more. The conference will also include vendors from across the country in the associated Exhibit Hall, providing consultation and products for interested attendees. The full educational program, registration, and hotel information will be available at https://rwc340b.org in early January 2026. To access rwc340b.org, you may need to turn off your VPN.
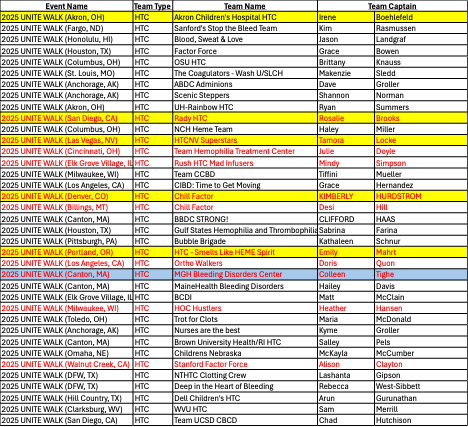
2025 Unite Walk Wall of Walkers Update
by Karen Bowe-Hause, Director of MCR
In collaboration with the National Bleeding Disorders Foundation (NBDF), the Hemophilia Alliance was proud to sponsor the 5th Annual Unite Walk Wall of Walkers campaign, designed to inspire increased partnership between our member Hemophilia Treatment Centers & their local Chapter affiliates. The Alliance would like to congratulate the 2025 Wall of Walkers Top 5 Hemophilia Treatment Center teams, as well as our Rookie of the Year team, who have all earned top fundraising status for the Unite for Bleeding Disorders Walk in their assigned market! HTC teams collectively raised almost $38,000 in 2025 to support local chapters—a remarkable accomplishment that highlights the power of partnership. Each of the 5 top HTC teams and our Rookie HTC team will receive a $1,500 scholarship that is intended to be used to send a staff member to the 2026 NBDF Bleeding Disorders Conference that will be held in Orlando, FL from August 13th – 15th.
The 5 winning teams and Rookie of the Year are:
- HTC – Smells Like HEME Spirit – (Team Captain – Emily Mahrt) – Oregon Health and Science University Hemophilia Center, in support of the Pacific Northwest Bleeding Disorders Foundation
- HTCNV Superstars – (Team Captain – Tamora Locke) – Hemophilia and Thrombosis Center Nevada in support of the Nevada Chapter of NBDF
- Rady Children’s HTC – (Team Captain – Rosalie Brooks) – Rady Children’s Hospital HTC in support of the Hemophilia Association of San Diego County
- Akron Children’s Hospital HTC – (Team Captain – Irene Boehlefeld) – Akron Children’s Hospital in support of Greater Ohio Bleeding Disorders
- Chill Factor – (Team Captain – Kimberly Hurdstrom) – University of Colorado Hemophilia and Thrombosis Center in support of Colorado Chapter, National Bleeding Disorders Foundation
- Our Rookie of the Year and First-time entrant – MGH Bleeding Disorder Center – Team Captain – Colleen Tighe) – Mass General Hemophilia and Thrombosis Clinic in support of New England Hemophilia Association.
Additionally, please plan on joining the virtual 2025 Unite for Bleeding Disorders Awards Ceremony on Tuesday, December 16th, at 2 PM ET, hosted by NBDF, to celebrate the incredible achievements of the 2025 walk season (Zoom link to join here).
- Yellow highlight = Highest fundraising team in each market group
- Blue highlight = Rookie of the Year team
- Red text = Teams that raised $1,000+ for the Wall of Walkers
Have a story you want to share or a topic you would like us to cover?