All members have the option of attending our various HTC-centric events which include:

Date: Friday, November 17, 2023
Time: 1:00pm – 2:00pm ET
TOPIC:
Clinical review of the Jivi® PROTECT VIII Trial and insights from HEM-POWR: A Real-World Evidence (RWE) Study of Jivi®
OVERVIEW:
This session includes key information about Jivi including, safety and efficacy data from the clinical trials, step-wise dosing and Jivi RWE Data from HEM-POWR, a multinational,open-label, prospective, non-interventional, multicenter cohort study of Jivi®.
There will also be a review of the Q4 2023 340B pricing for the Bayer Hemophilia A portfolio under the Hemophilia Alliance Contract.
TARGET AUDIENCE:
All HTC Staff
SPEAKER:

Mark T. Reding, MD.
Director, Center for Bleeding and Clotting Disorders
Professor of Medicine, Division of Hematology, Oncology, and Transplantation
University of Minnesota
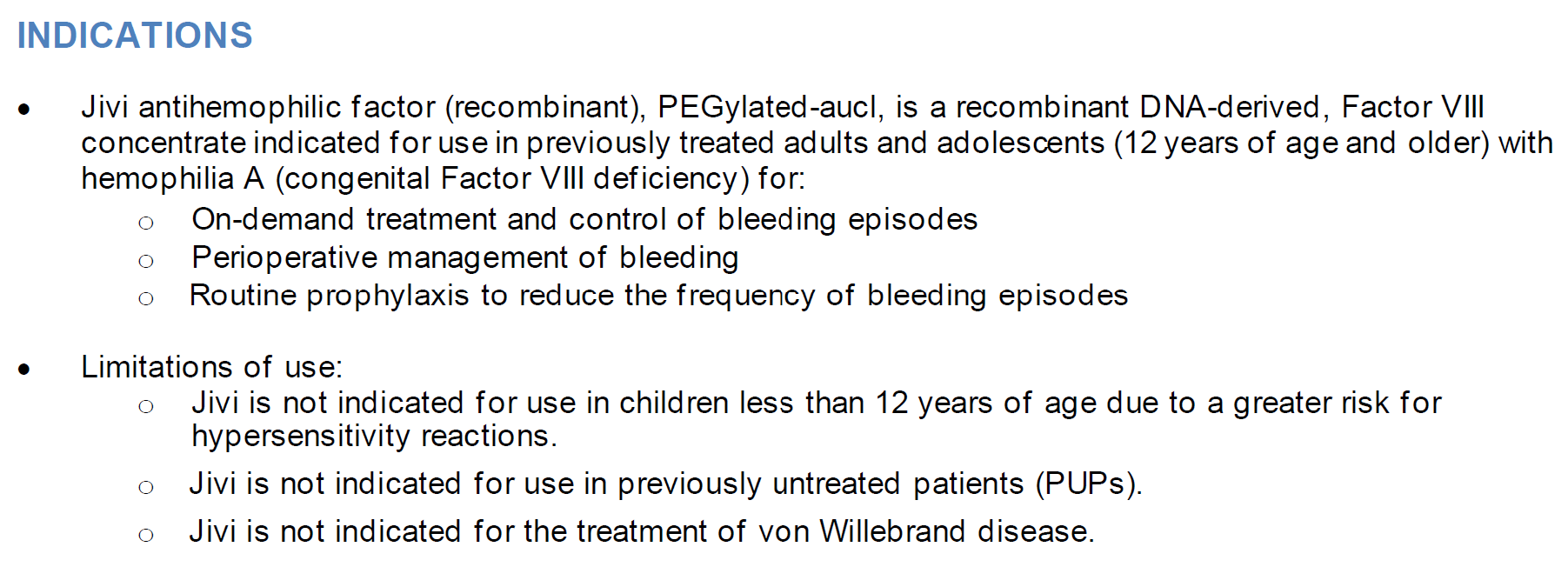

For additional important risk and use information, please see the full Prescribing Information for Jivi.
You are encouraged to report negative side effects or quality complaints of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.