In This Issue...
Have a story you want to share or a topic you would like us to cover?
Jeff Weighs In
Advocacy and Legal Update
Appropriations Measure is Signed Into Law
by Artemis Policy Group
In early February, Congress passed and the President signed into law a package (the Consolidated Appropriations Act of 2026, or CAA 2026) that provides funding for the federal health agencies for the remainder of the fiscal year. The law includes continued level funding for the two hemophilia programs at the Centers for Disease Control and Prevention (CDC) and the hemophilia program at the Health Resources and Services Administration (HRSA).
The CAA 2026 contains a number of substantive health policies in addition to appropriations language. While a few of the new policies could be relevant to HTCs, none raise significant concerns for the Hemophilia Alliance or its members. The new policies include:
- PBM reform provisions (narrower than in past bills), including:
- With respect to Medicare Part D only – require PBMs to pass through certain rebates and discounts to plan sponsors, and allow PBM to charge only flat administrative fees for their services.
- Require PBMs to report at least annually to plan sponsors and HHS regarding contracts, rebates, drugs dispensed, use of copay assistance, etc.
- Require PBMs under contract with ERISA plans to pass through drug rebates to the plan issuers.
- The Accelerating Kids’ Access to Care Act – policy supported by the Hemophilia Alliance that makes it easier for children with Medicaid or CHIP coverage to access providers across state lines.
- Extension of current Medicare telehealth flexibilities until December 31, 2027.
- Assuring pharmacy access and choice (“any willing provider”) for Medicare beneficiaries.
- Requiring a separate identification number and an attestation for each off-campus outpatient department of a provider by 2028.
340B Reform Efforts Remain Quiet
The CAA 2026 – the likeliest vehicle for 340B reform efforts – contained no provisions aimed at 340B or covered entities. Oversight and legal wrangling, however, continue. On February 1st, Senator Cassidy (chair of the Senate Health, Education, Labor and Pensions Committee) sent a lengthy letter asking for detailed information from Apexus, the 340B prime vendor, about its revenue streams and business practices. Also in February, HRSA indicated it would once again explore a 340B rebate model (the agency abandoned an earlier proposed demonstration model following litigation initiated by hospitals).
eAPTC Extension Appears Dead
On January 8, the House of Representatives passed legislation which would extend the ACA enhanced tax credits for an additional three years. Senate negotiators, however, have been unable to reach agreement on a path forward to extend the enhanced tax credits. Lawmakers and Hill watchers alike believe there is little chance that any measure will be enacted.
Update on Medicare Reimbursement Issues for Some Bleeding Disorders Treatments
Last December’s newsletter reported that certain member HTCs had received denials from Medicare for claims relating to newer non-factor hemophilia treatments that are administered by injection (e.g., Hympavzi, Qfitlia, and Alhemo). The problem apparently arose because local MACs (Medicare Administrative Contractors, the entities that pay Part A and B claims), had not updated their Part B clotting factor billing and coding articles to include the new treatments.
CMS’s January 2026 updates to its Medicare Part B payment files will hopefully resolve this issue. The updated files reflect all the new hemophilia injectables as clotting factors. At least one MAC (Noridian) has updated its Hemophilia Clotting Factor Billing page to conform to the CMS payment files. Please reach out to your Hemophilia Alliance MCR team member if you are still encountering Medicare reimbursement issues for the new hemophilia products.
Member and Community Relations Update
The MCR Team Is Here to Support Your HTC with Letters of Agreement, Single Case Agreements, and Gene Therapy Reimbursement
by The Member & Community Relations Team
The Member and Community Relations (MCR) team works closely with our member Hemophilia Treatment Centers (HTCs), ensuring that member HTCs have the expertise, resources, and public support to sustain their integrated clinical and pharmacy services for individuals with bleeding and clotting disorders.
Typically, the MCR team identifies opportunities for HTCs to dispense medications directly to patients through insurance payer and PBM contract negotiations, or through the Hemophilia Alliance Network Services (HANS). However, as treatment options and payer policies continue to evolve, member HTCs are facing new challenges in dispensing to their patients through their 340B programs. In these circumstances, Letters of Agreement (LOAs) or Single Case Agreements (SCAs) can be a path forward for HTC 340B programs to dispense to their patients, especially when it comes to self-insured plans.
Here is one recent example. A member HTC notified Hemophilia Alliance staff about a payer issue that would have resulted in one of their long-time hemophilia patients being forced to discontinue use of the HTC pharmacy. Working with the HTC, the MCR was able to identify the broker who worked with the patient’s employer and the payer to get an exception for the HTC to continue to service the patient from the HTC pharmacy. The payer and HTC are currently working to formalize this arrangement in an LOA.
In another recent case, a member HTC had a patient whose employer incorporated an alternative funding program (AFP) on their self-insured plan. The Hemophilia Alliance had previously worked with the HTC to establish an LOA with that employer, allowing the HTC’s 340B pharmacy to dispense to the patient – while controlling costs for the patient’s employer. When the employer added the AFP to their plan for 2025, the employer opted against applying the AFP to this patient’s life-saving bleeding disorder medications, and the patient continued to access his medications through the HTC pursuant to the LOA. Midway through the year, the patient expressed an interest in receiving gene therapy. Hemophilia Alliance staff initiated conversations with the broker of record for the employer; once it was determined the patient was a clinically appropriate candidate for gene therapy, the MCR, broker, and employer opened up dialog on a path forward for the patient. That path eventually led to a SCA with the HTC that outlined the reimbursement rate, the claims submission parameters (within 30 but no later than 45 days from the date of service), and payment parameters (timely but no greater than 30 days). The HTC submitted its claim in the afternoon after the infusion occurred and received payment in full six (6) days later.
These examples highlight how the Hemophilia Alliance and the MCR team are committed to partnering with your center so you can stay focused on what matters most – serving your patients. Whether your team needs assistance with an SCA, LOA, or a gene therapy case, the MCR team’s goal is to ensure your Center has the tools and support needed to secure timely patient access and program sustainability through 340B pharmacy dispensations. We can help you meet your payer challenges with confidence and a smile. 😊 We work for you!
Linda Gammage Social Worker Planning Committee Member Needed
by Jazzmine Brown, Director of MCR
The Hemophilia Alliance seeks to fill one vacancy on the Planning Committee for the Linda Gammage Social Worker Conference. Candidates are required to be social workers currently employed at a federally supported HTC that is a member of the Hemophilia Alliance. This volunteer opportunity involves working collaboratively with other committee members to identify appropriate topics, recruit speakers and plan/evaluate programs. Committee members participate in conference calls and one in-person planning meeting per year in early fall (if needed). The role generally requires a commitment of about two hours per month with the term being three years. Committee members are of course guaranteed a spot to attend the meeting, which usually occurs in late February.
Application Requirements: Interested applicants should submit the following:
- A current CV/resume;
- A letter of interest outlining your motivation and relevant experience;
- A letter of support from your HTC supervisor or medical director; and
- A letter of recommendation from a community professional familiar with your work and contributions.
Interested individuals should submit all application requirements to Jazzmine Brown at jazzmine@hemoalliance.org by Friday, March 6th.
5th Annual Pharmacist CE Meeting – The Best Yet!
by The MCR Team
[Click on image for larger version]
The Hemophilia Alliance 5th Annual Pharmacist CE Meeting was held January 21-23 in Tampa, Florida at the Current Hotel. In attendance were 54 pharmacists in-person and another 32 pharmacists and pharmacy technicians joining virtually. The faculty was outstanding, with attendees reporting “this may have been the best conference yet”!
Thank you to Takeda and The Alliance Pharmacy (TAP) for sponsoring this event. Takeda and TAP have sponsored every Pharmacist CE meeting for the last 5 years! Thank you, too, to the Indiana Pharmacy Association for reviewing 12 faculty presentations which can be submitted for ACPE credits for the next three years. This brings the total to 36 active CE credits available for home study for pharmacists and technicians. A grand total of 62 CE credits have been provided over the last 5 years. This is a marvelous benefit that is exclusive to our Hemophilia Alliance members.
The Pharmacists CE Planning Committee worked diligently to build another strong program. With the pending retirement of Mark Plencner, Heidi Lane, Angie Blue, and Theresa Parker joined veteran committee members Angela Kellum, Dana Smith, Stevan Mizimakoski, Matt Debrine, and Mark. The team worked hard to pull the program together in record time.
Topics covered during the conference included: sickle cell disease, AI in the pharmacy, patient assistance programs, von Willebrand disease diagnosis and therapies, inhibitors, new and emerging therapies in hemophilia, physical therapist-pharmacist collaboration, reproductive health for women with bleeding disorders, and autoimmune hematology.
Thursday’s dinner at the Rusty Pelican included a nice tribute to retiring Mark Plencner from both Jeff Blake and Stevan Mizimakoski which included a video presentation highlighting family and HA team activities.
All the substantive video presentations from this meeting are posted on the Hemophilia Alliance website in the document portal. Do yourself a favor and view the presentations. It will be well worth your effort. You will be impressed with the knowledge and expertise that is shared by members of our HTC community.
Information for submitting CE credits for home study is available on the Hemophilia Alliance website under the Document Portal section. Please note a login is required. If you are a member HTC of Hemophilia Alliance but don’t have a login, click here to request for one.
Program development is underway for 2027. Save the date: February 24-26, 2027. We look forward to seeing you then!
Administration and Operations Update
Annual Savings Letters and Member Dues – Be on the Lookout!
by The Admin & Ops Team
We are implementing a new process this year. We will be distributing Members’ Annual Savings letters alongside the invoices for annual member dues. Both the Savings Letters and member dues are based on a 12-month reporting period from Q4 2024 to Q3 2025.
The Annual Member Dues Structure is based on units of product dispensed:
- Start-up/Small: $1,200 per year: HTCs that have not yet dispensed bleeding disorders products, or have sold fewer than 5 million units within the past 12 months.
- Medium: $6,000 per year: HTCs with sales exceeding 5 million but less than 10 million units in the past 12 months.
- Large: $10,800 per year: HTCs with sales exceeding 10 million units in the past 12 months.
If you have any questions, please contact Jennifer Anders at jennifer@hemoalliance.org.
MPBA 2026 Meeting Recap: Strengthening the Future of HTCs Through Leadership, Strategy, and Collaboration
by The Admin & Ops Team
The Hemophilia Alliance kicked off the year with the Medical Provider & Business Administrator (MPBA) Meeting, held January 11–13 in Newport Beach, CA. Member HTC clinical and business leaders from across the country gathered for this Alliance-funded program to strengthen leadership, improve operations, and foster collaboration.
[Click on image for larger version]
The program opened with an optional pre-conference featuring a foundational session on HTC and 340B essentials, paired with a more advanced topic highlighting the gold standard for HTC–pharmacy integration, Building an Inhouse Pharmacy, before participants transitioned into the main agenda.
Program Highlights and Participant Feedback
Day 1
- HTC Models & Innovation: A valuable look at the range of center types and creative approaches to care delivery.
- Patient Support Services: Meaningful insights on documentation, compliance, and patient centered service models.
- Leadership Essentials: Invited speaker, Paula Mulford, delivered a well-received session outlining practical, memorable leadership strategies that could be implemented immediately.
- Staffing Strategies: Benchmarking across HTCs helped teams identify workforce needs and new approaches for growth.
- Payer & Pharmacy Challenges: This session’s thoughtful facilitation made a complex topic more approachable and validated shared challenges.
Day 2
- Washington Updates: Praised for clear summaries and forward-looking insights about the current policy landscape.
- Novel Therapies: Really valuable, appreciated the perspectives and identified areas for follow up.
- Advocacy: Attendees reported a stronger sense of what they can do locally and how to involve patients in advocacy efforts.
- Strategic Planning Tools: The business plan manual and exercises helped teams begin hands on planning during the session.
Many participants left with concrete next steps, including focusing on their HTC strategic/business plan, seeking more structured feedback from their teams, creating executive summaries/elevator pitches, and strengthening collaboration with local chapters on patient support initiatives.
“The MPBA Meeting has become one of the most valuable gatherings I attend each year. The depth of discussion, practical strategies, and open collaboration help me return to my HTC with fresh ideas and a clearer path forward.” — MPBA Past Attendee
As participation grows each year, it is increasingly clear that the MPBA Meeting is becoming an essential activity for every member HTC, offering unmatched opportunities for learning, strategy, and collaboration.
Save the Date – MPBA 2027: January 24–26
We look forward to bringing the community together again next year!
Notes From The Community
In Tribute to Dr. Lynn Malec
 We are saddened by the passing of Dr. Lynn Malec, a respected hematologist, valued contributor to the bleeding disorders community, and an avid supporter of the Hemophilia Alliance.
We are saddened by the passing of Dr. Lynn Malec, a respected hematologist, valued contributor to the bleeding disorders community, and an avid supporter of the Hemophilia Alliance.
Dr. Malec was an extraordinary physician, researcher, and advocate whose impact reached far beyond her professional accomplishments. She dedicated her life to improving care, advancing access to care, and centering the voices of patients and families. Those who had the privilege of working alongside Lynn knew her as a brilliant clinician, a generous mentor, and a compassionate leader who brought both rigor and humanity to everything she did. Her influence will live on through the countless lives she touched, the colleagues she inspired, and the community she helped strengthen.
Our hearts are with Dr. Malec’s family, friends, colleagues, and the many communities she served. We honor her legacy by reaffirming our commitment to the work she championed—advancing access to care, improving care, and ensuring that patients and families remain at the center of everything we do.
Spring 340B Grantees Conference
by Jeff Blake, Heidi Lane, and Jennifer Borrillo
Hemophilia Alliance senior leadership is excited to share that for the first time we will be coordinating efforts with the Ryan White Clinics for 340B Access (RWC-340B) on planning sessions at their Spring 2026 Grantees Conference for the first time.
RWC-340B and their conference partners are inviting all Federal Grantees, including organizations such as Ryan White Clinics, Federally Qualified Health Centers (FQHCs), and specialty clinics including Tribal, Black Lung, and HTCs, to attend the conference. The conference curriculum holds value for every grantee and staff member, regardless of role or background.
The Spring 2026 Grantees Conference will take place in Houston, Texas, from May 28-May 30, 2026, at the Hyatt Regency Houston Downtown. Preconference education will begin on May 28th, with the nationally acclaimed 340B University, sponsored by Apexus, the Prime 340B Vendor. Two full days of plenary and breakout sessions will follow, covering issues of concern to grantees, such as fiscal management, audit and compliance, advocacy, pharmacy management, use of TPAs, and more. The conference will also include vendors from across the country in the associated Exhibit Hall, providing consultation and products for interested attendees. The full educational program, registration, and hotel information is available here. To access rwc340b.org, you may need to turn off your VPN.
NBDF Issues Statement on Gene Therapy
by Artemis Policy Group
The National Bleeding Disorders Foundation published a statement reiterating its commitment to shared decision-making and evidence-based access in gene therapy. NBDF recognizes that the current coverage landscape can create challenges for individuals interested in gene therapy, as well as for their treating clinicians and HTCs. The statement urges payers to allow appropriate consideration of gene therapy when it is prescribed consistent with its labeled indication and with providers’ clinical judgment.
Central Virginia Center for Coagulation Disorders Offers New Clinic
by Kelly Waters, Director of MCR
VCU’s Central Virginia Center for Coagulation Disorders (CVCCD) has expanded access to adult bleeding disorder care across the region by offering a dedicated clinic at VCU Health at William & Mary. This expansion brings lifespan care to the Tidewater region for the first time, helping bridge the gap between pediatric and adult services while significantly reducing the several hours of travel previously required to access care in Richmond.
The new quarterly satellite clinic provides comprehensive, multidisciplinary care, including infusions for the prevention of bleeding episodes, along with support from social workers, genetic counselors, and physical therapists. This team-based approach ensures that patients’ medical needs are addressed while also supporting the emotional, social, and financial challenges they may face.
Click here to watch media coverage about the clinic’s opening and click here to read more about VCU’s expanded adult bleeding disorder care.
Tool for Pharmacies
by Angela Kellum, PharmD., Louisiana Center for Bleeding & Clotting Disorders
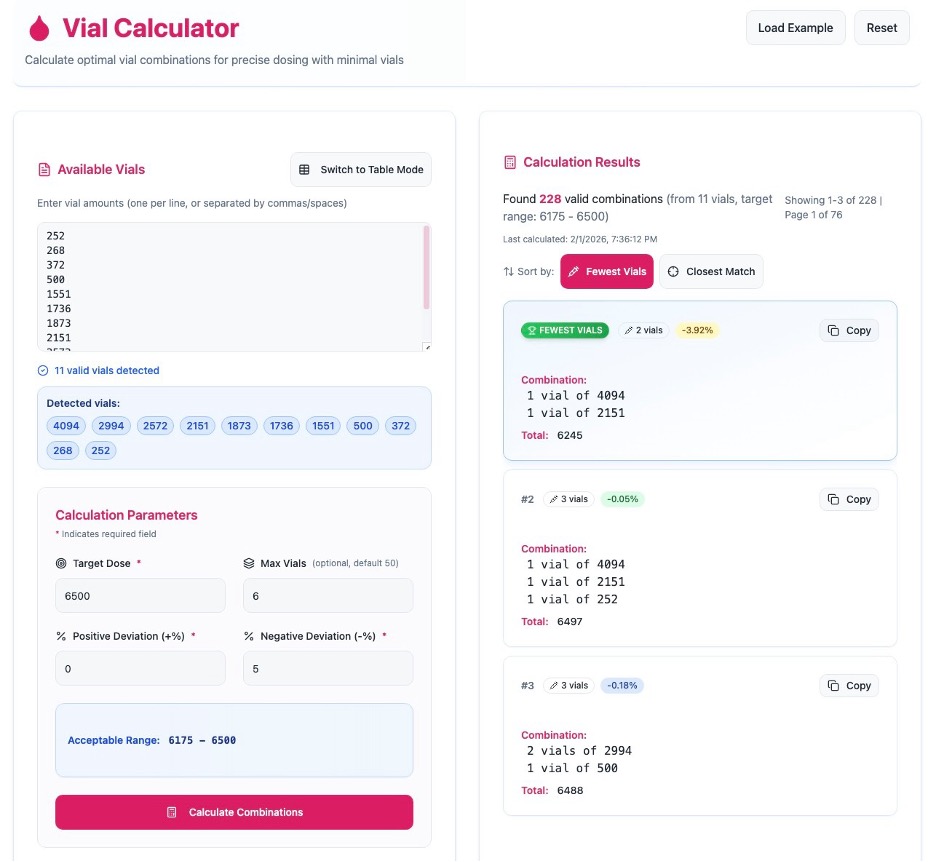
One step in our pharmacy workflow when dispensing clotting factor is determining which vial sizes to combine to meet a patient’s prescribed dose.
Background
Clotting factor products are unique in that the actual unit amount in each vial does not match the unit amount associated with the NDC. For example, a 4,000 unit vial may contain hundreds of units more or less than 4,000. Additionally, many patients require more than one vial per dose due to available vial sizes and individualized dosing parameters.
Importance
Pharmacies calculate vial combinations that meet the prescribed dose while staying within allowable deviation ranges. This matters not only clinically, but also financially. Factor costs are per unit, and cost containment is critical. Also, many pharmacies must comply with strict payor contract requirements, often within very tight ± percentage limits.
We also are mindful of patient convenience and possible adherence challenges and therefore want to minimize the number of vials that patients must store, reconstitute, draw up and infuse. This is especially important for pediatric patients and for those with fragile veins who may struggle with larger infusion volumes.
Challenge
Traditionally we relied on pen, paper, and a calculator. With so many possible vial combinations, this process was slow and often left us wondering whether we truly had found the best option.
Solution
One day while I was working remotely, I asked my son, Samuel—who happens to be very good at math—to help me figure out the optimal vial combination for a patient’s order. He found the answer quickly. Then, later he developed a program that could do the calculation for me! That program can now be found at: https://vialcalculator.com/.
We now use this program almost every day in our in-house 340B pharmacy. I also rely on it when placing large monthly replenishment orders for our contract pharmacy. It has been shared with HTC pharmacy colleagues and hospitals across the state and across the country, and the reaction is always the same—relief that manual calculations are no longer necessary!
How it works
The program is housed on its own website: https://vialcalculator.com/
Using the program takes just 3 simple steps:
- Enter vial inventory
Free-type or copy and paste available vial inventory directly into the program—there is no need to remove alpha characters or clean up cells. If inventory is limited for specific vial sizes, “Switch to Text Mode” allows entry of exact vial counts. - Enter dosing parameters
Input the prescribed dose and allowable percent deviation (such as ±10% or +0/−5%). An optional field allows you to set a maximum number of vials per dose or dispensation. The calculator can accommodate up to 100 vials, which is especially helpful for large replenishment orders. - Calculate combinations
With one click, the program instantly generates optimal vial combinations.- Results can be sorted by Fewest Vials or Optimal Combinations
- The selected combination can be copied directly into an ordering email, printed for inventory pulling, or saved for documentation
Final thoughts
This tool has become an integral part of our pharmacy workflow, and it has improved efficiency, confidence, and consistency in factor dispensing. I encourage you to share it with your pharmacy teams and contract pharmacies. I’m confident they’ll find it just as helpful.
For feedback or suggestions, feel free to email Angela Kellum at: akellum1@tulane.edu.
Have a story you want to share or a topic you would like us to cover?